Manufacturer of bladder drug Elmiron fails to warn of reactions signaling significant eye damage
 Medications are meant to bring relief and peace of mind, but when your treatment begins to attack unrelated areas of your body, that peace of mind can be compromised. In June 2020, the Food and Drug Administration (‘FDA’) issued a Labeling Change related to the prescription drug Elmiron used for a bladder condition. This label change gave warning that Elmiron can cause issues with eyesight. Although there has been no recall, users were advised to get regular eye exams and stop using it if they notice problems with their vision.
Medications are meant to bring relief and peace of mind, but when your treatment begins to attack unrelated areas of your body, that peace of mind can be compromised. In June 2020, the Food and Drug Administration (‘FDA’) issued a Labeling Change related to the prescription drug Elmiron used for a bladder condition. This label change gave warning that Elmiron can cause issues with eyesight. Although there has been no recall, users were advised to get regular eye exams and stop using it if they notice problems with their vision.
Elmiron is used to treat pain and discomfort from interstitial cystitis (‘IC’), a painful (and common) bladder condition which is difficult to treat and often mistaken for urinary tract infections. Elmiron is believed to help with the pain from this condition by attaching to the bladder wall and creating a barrier to prevent more damage. Elmiron has been prescribed to treat this condition for decades and is the only drug approved by the FDA to do so.
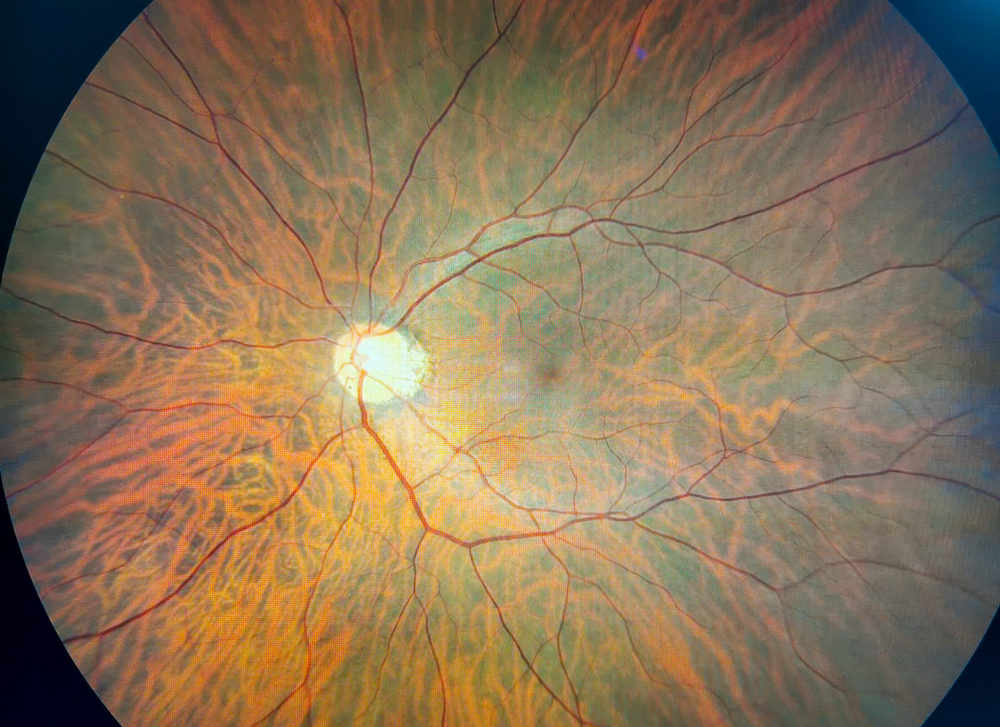
After a troubling study at Emory University in 2018, researchers wrote a letter to the Journal of Urology stating they found that long term use of Elmiron can cause serious damage to the retina. This “pigmentary maculopathy” comes from injury to the outer layer of the retina. Physicians at Kaiser Permanente took this warning seriously and reviewed medical records of over four million of their patients and were able to actually study 91 of them who had been taking Elmiron over a long period of time. Around one-quarter of the patients examined were found to have significant damage to the retina which had previously—and unfortunately—been attributed to other diseases that affect the eyes.
The negative effects of the drug are usually only noticed after long term use and appear to be unique to people who take Elmiron. The eye damage symptoms can include prolonged light to dark adaptation, blurry vision, difficulty reading, metamorphopsia (lines look curvy), central vision loss, loss of vision detail, dimming of vision, blind spots, floaters, spots, impaired color vision, difficulty with near vision, or even blindness.
Again, despite these observations, there has been no recall and Elmiron remains on the market. Even following these studies, the manufacturers of Elmiron did not warn its patients about the detrimental effects of their drug. It is worth noting that, Janssen issued warnings in Canada about Elmiron causing eye damage as early as September 2019. Research has shown that damage can be halted if someone stops taking the medication, but since Elmiron can take several months to take effect many patients can end up taking it for several months, or even years with the damage being done before the cause is determined.
This is not the first time that Janssen Pharmaceuticals (the manufacturer of Elmiron) and Johnson & Johnson (owner of Janssen), were found to be manufacturing or distributing harmful products. Dangerous drug and medical product attorneys have reached settlements with respect to illegal marketing of a dangerous dementia drug Risperdal, erosion of vaginal mesh implants, cancer risk from baby powder, and most notably, the debilitating opioid crisis in the United States.
Have you or a loved one have experienced any of the above symptoms after taking Elmiron, or any dangerous medication? Contact the experienced dangerous drug attorneys at Neumann Law Group for a free consultation.